Hemp Testing Solutions

Unmatched customer service
Results released 24/7
National and state-specific compliance panels
Groundbreaking research and development

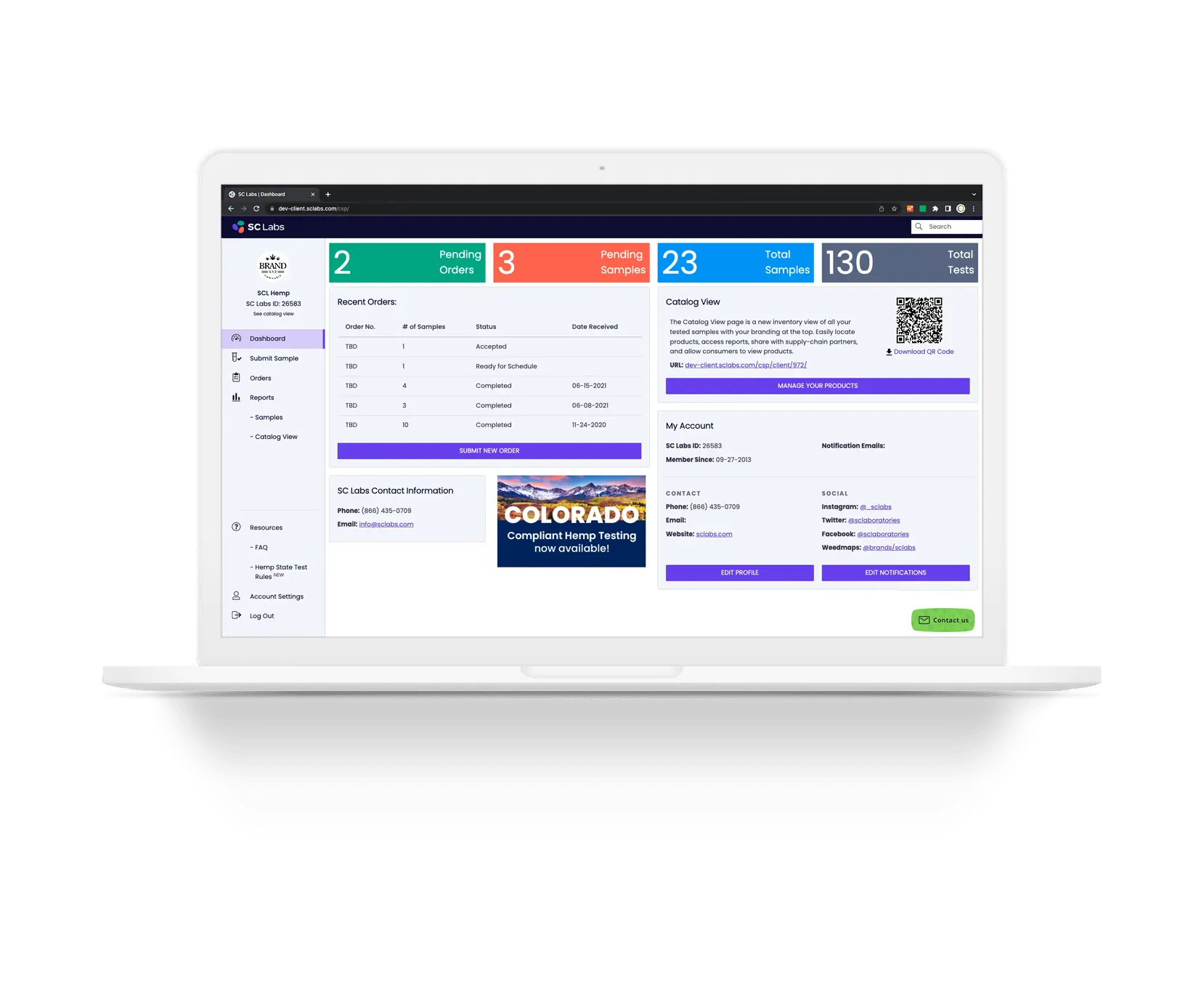
Verify compliance with federal and state requirements
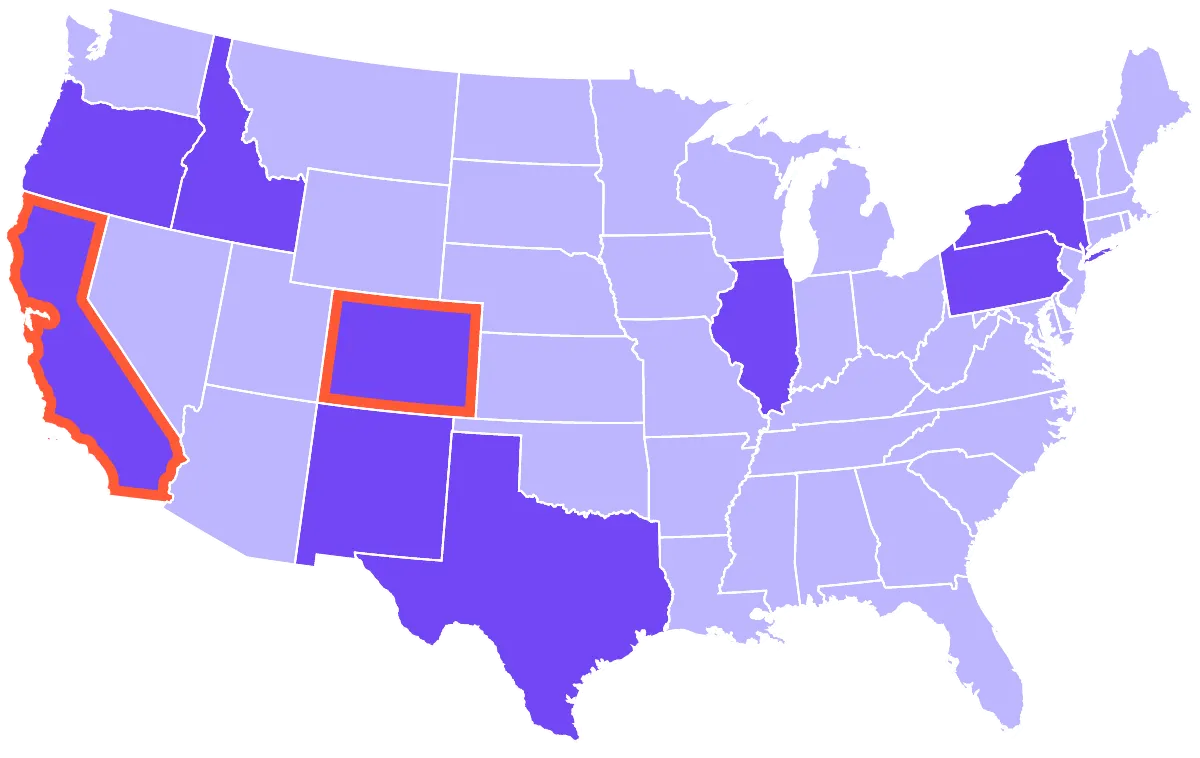
SC Labs offers a national hemp testing package to verify compliance with USDA requirements. For operators in California and Colorado, we offer state-specific testing packages. And SC Labs is a registered hemp testing provider in every state that requires registration.
California, Colorado
California, Colorado, Idaho, Illinois, New Mexico, New York, Oregon, Pennsylvania, Texas
As permitted by each state.
Avoid costly recalls and build customer loyalty
SC Labs offers a broad range of tests from early-stage R&D product development to quality assurance tests during the production process.
New CBD & THC Beverage Testing ensures compliance, purity, and potency in every sip.

Demonstrate your commitment to product quality and safety with QR codes for test data that is publically available
How and where you use your test data is easy to manage with SC Labs’ secure Client Service Portal (CSP).