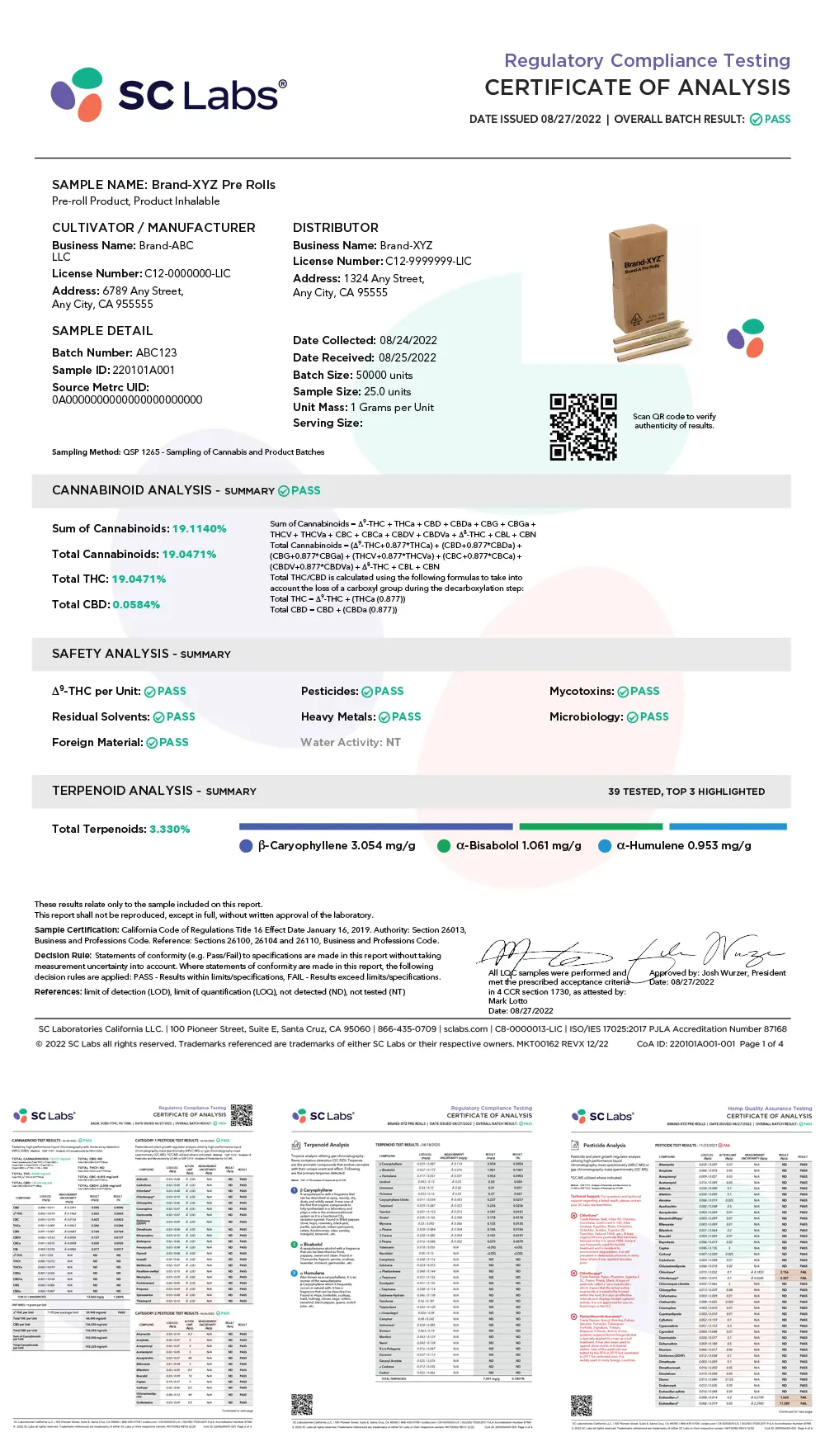
Make informed decisions quickly with clearly defined test results. Our easy-to-read Certificate of Analysis (COA) provides clear, detailed information—allowing you to make better, faster decisions about product quality and consistency.
What tests will you find on the COA? That depends upon whether the product is intended for sale and tested for compliance or if it’s being tested for research and development (R&D) or quality assurance/quality control (QA/QC) purposes. Our COAs are dynamically built to support either scenario.
Have a look for yourself
Take a minute to explore the interactive graphic pictured here. Hover over the markers to learn what each section means and how it applies to the products we test. While the following images show a compliance COA, our QA/QC version will incorporate similar features.